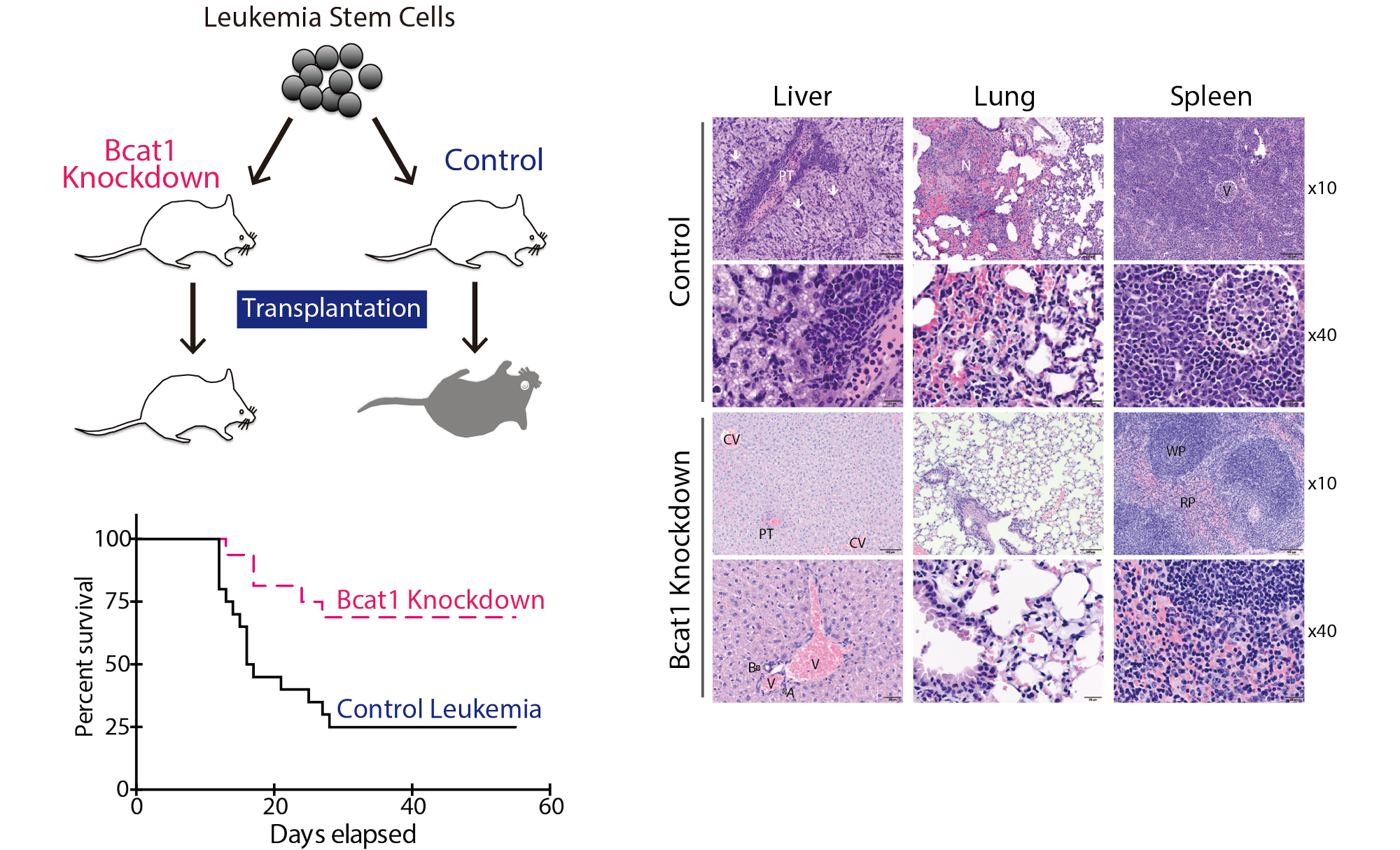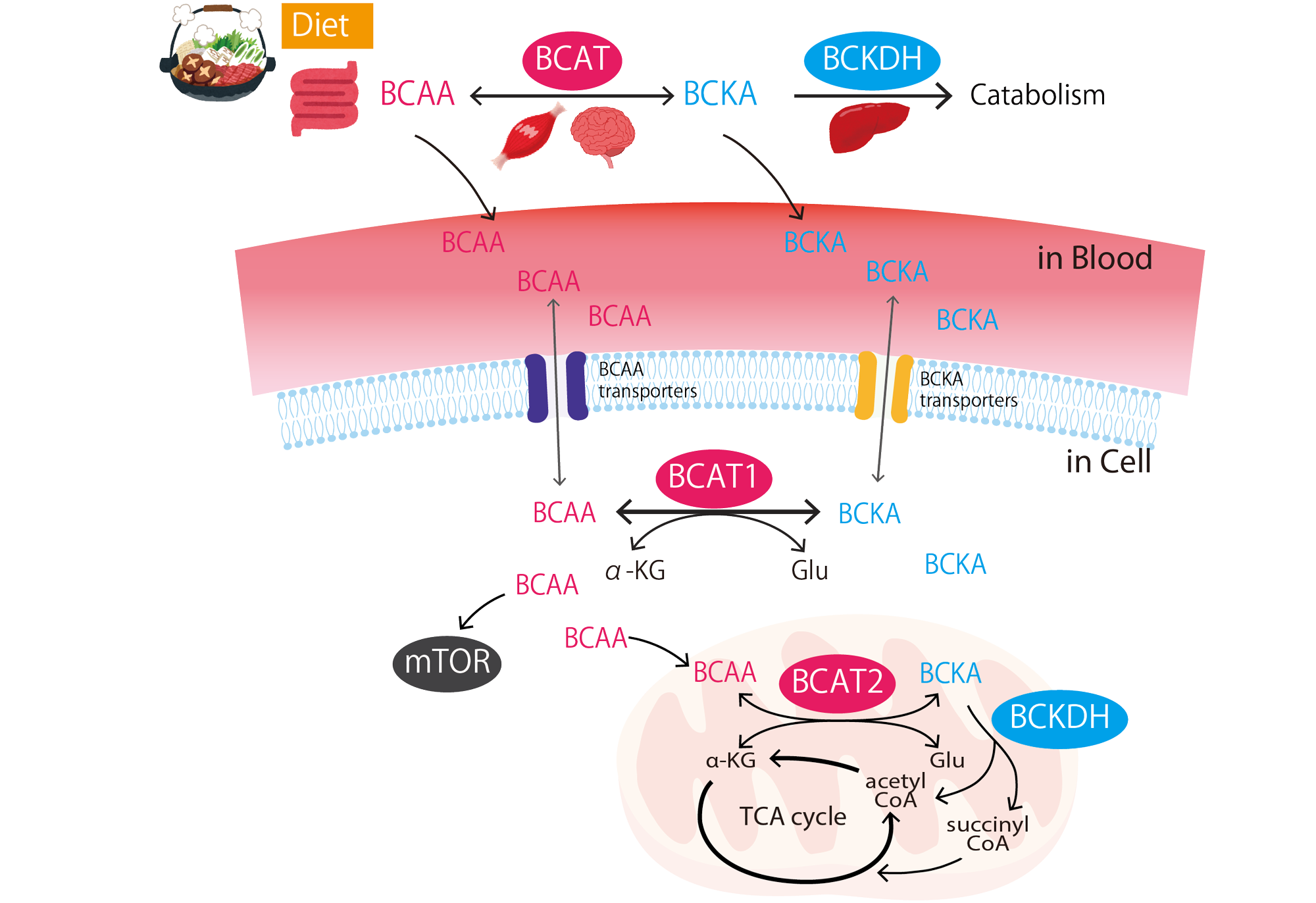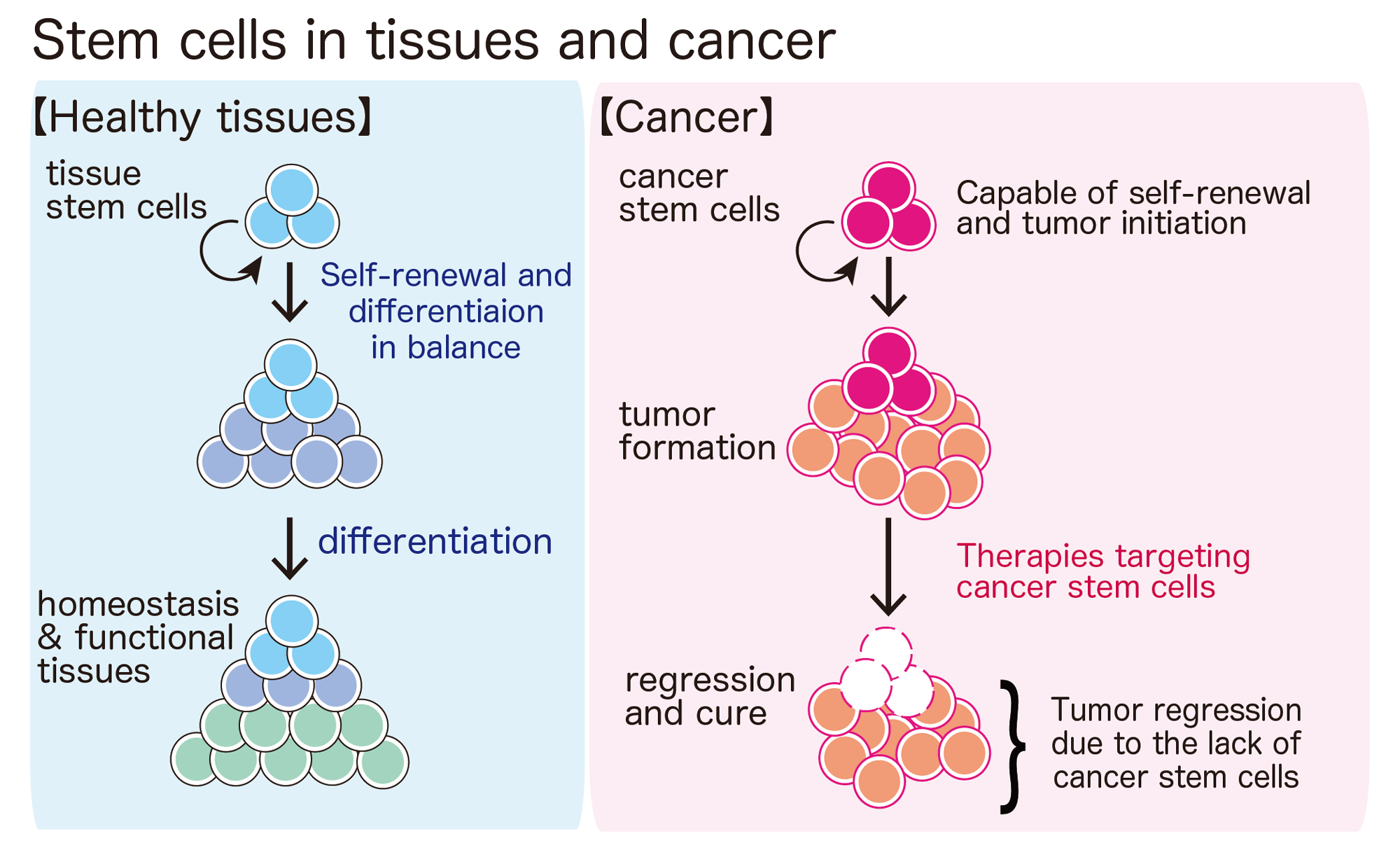
Tissue stem cells in aging and regenerative medicine
Self-renewal is an incredible cellular ability found only in stem cells. With the self-renewing potential, a stem cell can maintain an entire tissue with elaborate functions for a lifetime, such as blood, gut, skin, skeletal muscle — you name it.
Because stem cells are multi-potent, tissue stem cells can produce specialized cells that are required for the given tissue to function properly. For example, blood stem cells (or hematopoietic stem cells) can generate all kinds of blood cells found in the human body; these include, but are not limited to, red blood cells (erythrocytes) for oxygen distribution, platelets for blood coagulation, B- and T- lymphocytes for acquired immunity, and granulocytes and macrophages for innate immune responses. These cells are generated at high rates to replace old blood cells. Each day, 100 billion white cells, 200 billion red cells and 400 billion platelets are being produced. Collectively, it is almost a trillion per day, and all of these cells can originate from a single hematopoietic cell! Even more surprisingly, the massive cell production can last a lifetime because hematopoietic stem cells can generate themselves and increase their numbers without losing their multipotency, which we call self-renewing potential.
Because self-renewing stem cells can provide as many “functional” cells as needed, they not only maintain functioning tissues but also help to repair damaged tissues after injury. When we have a cut, platelets stop bleeding, immune cells prevent infections, and the cut is sealed to initiate the skin repair process. Dead cells are removed and new skin cells (keratinocytes) generated from epidermal stem cells start to populate the site of injury and eventually healing the wound. It would be very hard to survive if there was no way to repair damage after tissue injury. Also, as we get older, the capability of stem cell self-renewal declines in many tissues. Therefore, our stem cells’ ability to repair and regenerate is also essential for us to live a long and healthy life. In our lab, we study how self-renewal is regulated in tissue stem cells.